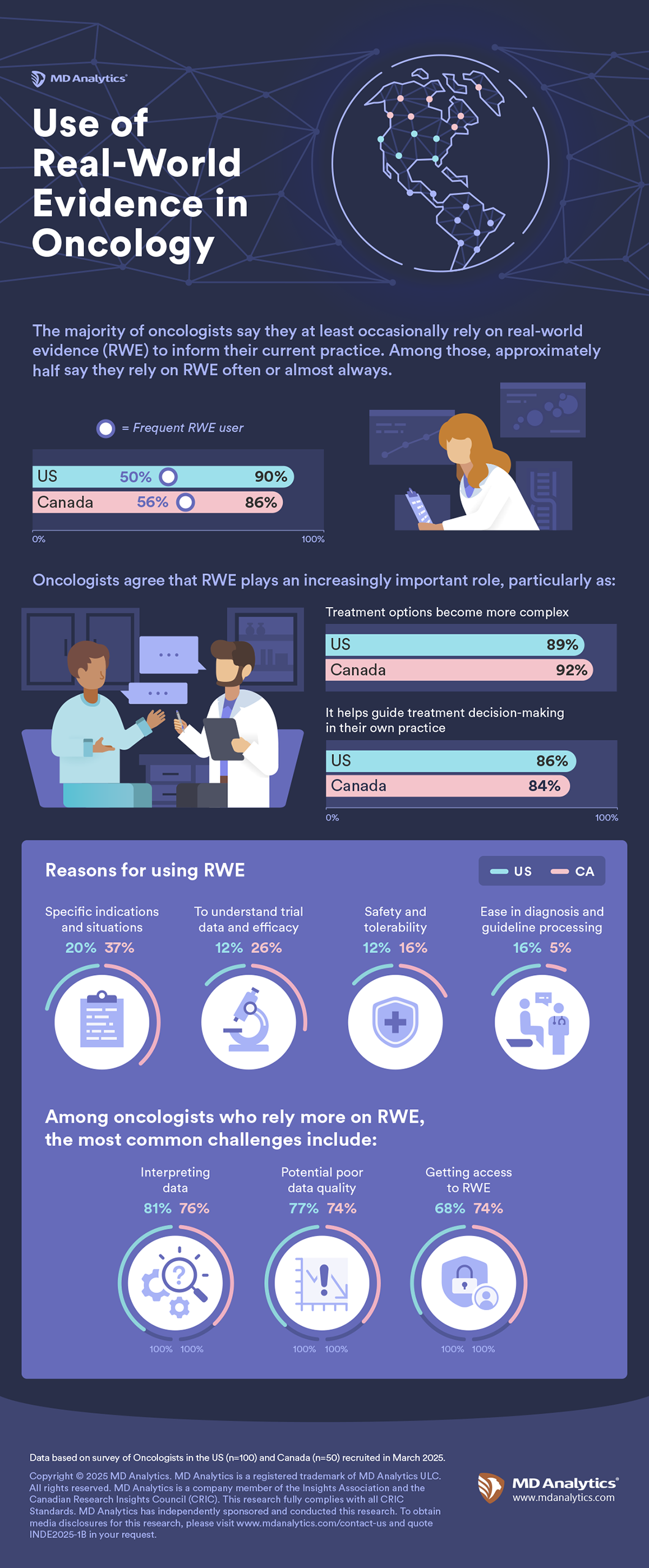
As oncology treatments have become more personalized and complex, the value of real-world evidence (RWE) has grown significantly. To better understand how oncologists are integrating RWE into their clinical decision-making, we surveyed 50 Canadian and 100 U.S.-based oncologists. The findings highlight both the rising importance of RWE in practice and the key areas where pharmaceutical companies can play a more active, supportive role.
Most surveyed oncologists – 86% in Canada and 90% in the U.S. – indicate they at least occasionally rely on RWE to inform their treatment decisions and among them, more than half of Canadian oncologists (56%) and half of U.S. oncologists (50%) say they rely on RWE often or always. This suggests real-world data has become an essential part of routine clinical decision-making and may represent an opportunity for pharmaceutical companies to ensure the RWE they generate is not only robust but also directly relevant to clinical practice.
Read More
Overall oncologists suggest this reliance on RWE is tied to the increasing complexity of oncology care. An overwhelming 92% of Canadian oncologists and 89% of U.S. oncologists agreed that RWE has become more important as treatment regimens have evolved and become more complicated. Furthermore, 84% of Canadian and 86% of U.S. respondents said RWE now plays an increasingly important role in their individual practice.
Oncologists cite a variety of reasons that go beyond efficacy for using RWE, with many turning to RWE to guide decision-making in specific clinical scenarios or indications that may not be fully addressed by trial data. Others use to evaluate safety and tolerability outside the controlled environment of clinical trials. A smaller yet meaningful group of clinicians also say RWE helps them process clinical guidelines and make more confident diagnostic decisions.
While increasingly important it is important to note the challenges oncologists face when working with RWE. Interpreting data emerged as a top concern, cited by 76% of Canadian and 81% of U.S. oncologists. Many also highlight potential challenges with quality of the data available (74% in Canada and 77% in the U.S). Access is also noted as a further barrier with nearly three-quarters of Canadian respondents and over two-thirds of U.S. respondents saying they face difficulty obtaining access to RWE.
For pharmaceutical stakeholders, these findings serve both as validation and a call to action. The demand for meaningful RWE is high, and the potential to make a clinical impact significant. But to maximize impact, pharma must invest in the clarity, quality, and accessibility of RWE resources. This means not only improving data methodologies and transparency, but also ensuring insights are delivered in a way that clinicians can understand and apply.
Past Studies
AI in Healthcare – Physicians Are Using Tools Like ChatGPT, But Concerns Remain
The use of AI in healthcare, such as generative tools like large language model (LLM) chatbots, has grown rapidly in over the past two years. Nearly all physicians have...
How Do Oncologists Really View Sales Reps and MSLs?
How Do Oncologists Really View Sales Reps and MSLs? In today’s highly competitive oncology market, meaningful interactions between healthcare professionals (HCPs),...
Leaders in Oncology
In oncology, competition is intense and innovation moves fast. Leading companies are not only developing groundbreaking therapies but also excelling in building...
Past Studies
Physicians are ready for AI. Are pharma companies ready to support them?
AI in Healthcare – Physicians Are Using Tools Like ChatGPT, But Concerns Remain
The use of AI in healthcare, such as generative tools like large language model (LLM) chatbots, has grown rapidly in over the past two years. Nearly all physicians have...
How Do Oncologists Really View Sales Reps and MSLs?
How Do Oncologists Really View Sales Reps and MSLs? In today’s highly competitive oncology market, meaningful interactions between healthcare professionals (HCPs),...
Leaders in Oncology
In oncology, competition is intense and innovation moves fast. Leading companies are not only developing groundbreaking therapies but also excelling in building...